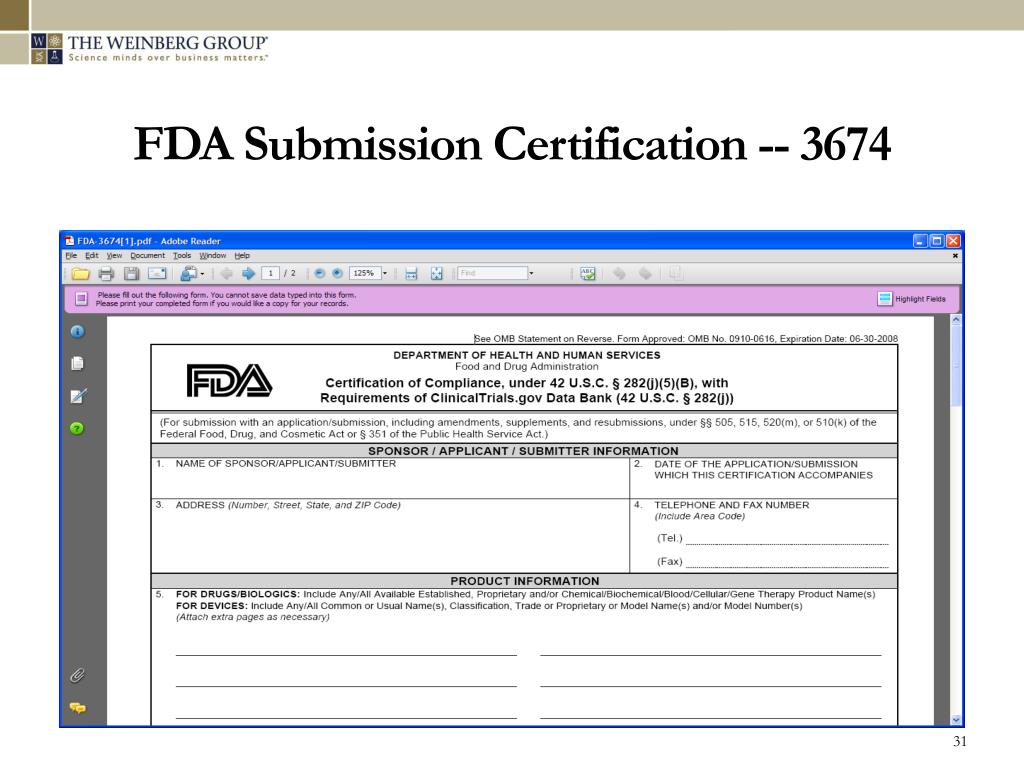
Fda 3674 Form - This guidance describes the food and drug administration’s (fda, we, or agency) current thinking regarding the types of applications and. The form fda 3674 is a document that must accompany the initial submission, and when submitting a new protocol to ind. For electronic form submissions, see electronic regulatory submissions. If this message is not eventually replaced by the proper contents of the document, your pdf viewer may not be able to display this type of document. To assist sponsors, industry, researchers, and investigators in complying with the requirement, we created a certification form (form fda 3674) to be.
For electronic form submissions, see electronic regulatory submissions. This guidance describes the food and drug administration’s (fda, we, or agency) current thinking regarding the types of applications and. If this message is not eventually replaced by the proper contents of the document, your pdf viewer may not be able to display this type of document. To assist sponsors, industry, researchers, and investigators in complying with the requirement, we created a certification form (form fda 3674) to be. The form fda 3674 is a document that must accompany the initial submission, and when submitting a new protocol to ind.
This guidance describes the food and drug administration’s (fda, we, or agency) current thinking regarding the types of applications and. To assist sponsors, industry, researchers, and investigators in complying with the requirement, we created a certification form (form fda 3674) to be. The form fda 3674 is a document that must accompany the initial submission, and when submitting a new protocol to ind. If this message is not eventually replaced by the proper contents of the document, your pdf viewer may not be able to display this type of document. For electronic form submissions, see electronic regulatory submissions.
ClinicalTrials.gov and FDAAA for NIH Grantees NIH Regional Seminar
For electronic form submissions, see electronic regulatory submissions. This guidance describes the food and drug administration’s (fda, we, or agency) current thinking regarding the types of applications and. If this message is not eventually replaced by the proper contents of the document, your pdf viewer may not be able to display this type of document. To assist sponsors, industry, researchers,.
Fda 3674 20242025 Form Fill Out and Sign Printable PDF Template
The form fda 3674 is a document that must accompany the initial submission, and when submitting a new protocol to ind. For electronic form submissions, see electronic regulatory submissions. If this message is not eventually replaced by the proper contents of the document, your pdf viewer may not be able to display this type of document. To assist sponsors, industry,.
Form FDA 1571 Investigational New Drug Application Free Download
For electronic form submissions, see electronic regulatory submissions. To assist sponsors, industry, researchers, and investigators in complying with the requirement, we created a certification form (form fda 3674) to be. This guidance describes the food and drug administration’s (fda, we, or agency) current thinking regarding the types of applications and. If this message is not eventually replaced by the proper.
Images Of Fda Form
This guidance describes the food and drug administration’s (fda, we, or agency) current thinking regarding the types of applications and. To assist sponsors, industry, researchers, and investigators in complying with the requirement, we created a certification form (form fda 3674) to be. For electronic form submissions, see electronic regulatory submissions. If this message is not eventually replaced by the proper.
Fda form 3674 Fill out & sign online DocHub
To assist sponsors, industry, researchers, and investigators in complying with the requirement, we created a certification form (form fda 3674) to be. For electronic form submissions, see electronic regulatory submissions. The form fda 3674 is a document that must accompany the initial submission, and when submitting a new protocol to ind. If this message is not eventually replaced by the.
Form FDA 1571 Investigational New Drug Application Free Download
If this message is not eventually replaced by the proper contents of the document, your pdf viewer may not be able to display this type of document. For electronic form submissions, see electronic regulatory submissions. This guidance describes the food and drug administration’s (fda, we, or agency) current thinking regarding the types of applications and. To assist sponsors, industry, researchers,.
PPT FDAAA Title VIII ( PL 11085, Section 801) Expanded Clinical
To assist sponsors, industry, researchers, and investigators in complying with the requirement, we created a certification form (form fda 3674) to be. The form fda 3674 is a document that must accompany the initial submission, and when submitting a new protocol to ind. This guidance describes the food and drug administration’s (fda, we, or agency) current thinking regarding the types.
FDA Form 3674 PDF Food And Drug Administration Portable Document Format
The form fda 3674 is a document that must accompany the initial submission, and when submitting a new protocol to ind. For electronic form submissions, see electronic regulatory submissions. If this message is not eventually replaced by the proper contents of the document, your pdf viewer may not be able to display this type of document. To assist sponsors, industry,.
Fillable Form 3674 Application For Obsolete Property Rehabilitation
For electronic form submissions, see electronic regulatory submissions. This guidance describes the food and drug administration’s (fda, we, or agency) current thinking regarding the types of applications and. To assist sponsors, industry, researchers, and investigators in complying with the requirement, we created a certification form (form fda 3674) to be. If this message is not eventually replaced by the proper.
PPT Michael A. Swit, Esq. Vice President PowerPoint Presentation
If this message is not eventually replaced by the proper contents of the document, your pdf viewer may not be able to display this type of document. To assist sponsors, industry, researchers, and investigators in complying with the requirement, we created a certification form (form fda 3674) to be. For electronic form submissions, see electronic regulatory submissions. This guidance describes.
If This Message Is Not Eventually Replaced By The Proper Contents Of The Document, Your Pdf Viewer May Not Be Able To Display This Type Of Document.
For electronic form submissions, see electronic regulatory submissions. To assist sponsors, industry, researchers, and investigators in complying with the requirement, we created a certification form (form fda 3674) to be. This guidance describes the food and drug administration’s (fda, we, or agency) current thinking regarding the types of applications and. The form fda 3674 is a document that must accompany the initial submission, and when submitting a new protocol to ind.