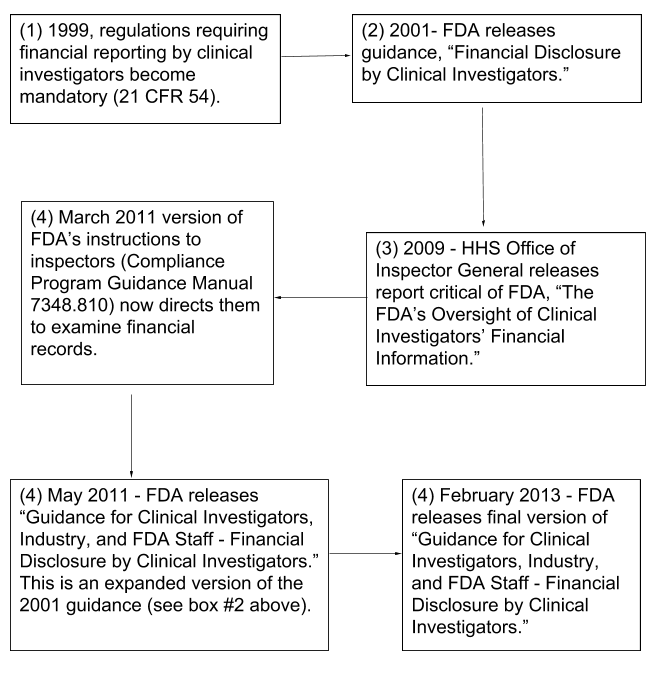
Fda Financial Disclosure Form - This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study. Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. This page provides links to commonly used clinical trial forms relevant to clinical trials. Everyone listed on the form fda. An applicant may consult with fda as to which clinical studies constitute “covered clinical studies” for purposes of complying with financial. For questions, refer to the fda guidance:. In compliance with the u.s.
This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. In compliance with the u.s. For questions, refer to the fda guidance:. Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study. Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. Everyone listed on the form fda. An applicant may consult with fda as to which clinical studies constitute “covered clinical studies” for purposes of complying with financial. This page provides links to commonly used clinical trial forms relevant to clinical trials.
Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study. This page provides links to commonly used clinical trial forms relevant to clinical trials. Everyone listed on the form fda. An applicant may consult with fda as to which clinical studies constitute “covered clinical studies” for purposes of complying with financial. This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. For questions, refer to the fda guidance:. In compliance with the u.s.
Financial Disclosure Form Template
For questions, refer to the fda guidance:. Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. In compliance with the u.s. An applicant may consult with fda as to which clinical studies constitute “covered clinical studies”.
Form FDA 3455 Disclosure Financial Interest and Arrangements of
This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. For questions, refer to the fda guidance:. This page provides links to commonly used clinical trial forms relevant to clinical trials. Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study. Everyone listed on.
Form FDA 3455 Disclosure Financial Interest and Arrangements of
In compliance with the u.s. An applicant may consult with fda as to which clinical studies constitute “covered clinical studies” for purposes of complying with financial. Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. Everyone listed on the form fda. For questions, refer to the fda guidance:.
FDA’s Requirements for Financial Disclosure Sharlin Consulting
This page provides links to commonly used clinical trial forms relevant to clinical trials. Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study. An applicant may consult with fda as to which clinical studies constitute “covered clinical studies” for purposes of complying with financial. Everyone listed on the form fda..
Form FDA 3627 A Guide for the Submission of Initial Reports Free Download
Everyone listed on the form fda. This page provides links to commonly used clinical trial forms relevant to clinical trials. Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. Fill out a separate financial disclosure form.
Financial Disclosure Form Template Fill Online, Printable, Fillable
In compliance with the u.s. Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study. Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. An applicant may consult with fda as to which clinical studies constitute “covered clinical studies” for purposes of complying with.
Form FDA 3410 Confidential Financial Disclosure Report for Special
This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. In compliance with the u.s. An applicant may consult with fda as to which clinical studies constitute “covered clinical studies” for purposes of complying with financial. Fill out a separate financial disclosure form for each entity that could be considered a sponsor for.
FDA Financial Disclosure Requirements for Clinical Investigators Ti…
For questions, refer to the fda guidance:. In compliance with the u.s. Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. Everyone listed on the form fda. This page provides links to commonly used clinical trial forms relevant to clinical trials.
Form FDA 3410 Confidential Financial Disclosure Report for Special
An applicant may consult with fda as to which clinical studies constitute “covered clinical studies” for purposes of complying with financial. This page provides links to commonly used clinical trial forms relevant to clinical trials. Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their. Everyone listed on the form fda. Fill out a.
PPT Office of Research UMDNJ School of Health Related Professions
In compliance with the u.s. For questions, refer to the fda guidance:. This page provides links to commonly used clinical trial forms relevant to clinical trials. This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. Fill out a separate financial disclosure form for each entity that could be considered a sponsor for.
In Compliance With The U.s.
Everyone listed on the form fda. For questions, refer to the fda guidance:. This form attests to the absence of financial interests, or discloses the nature of any financial arrangements. Code of federal regulations 21cfr54, clinical investigators are required to disclose to the study sponsor their.
An Applicant May Consult With Fda As To Which Clinical Studies Constitute “Covered Clinical Studies” For Purposes Of Complying With Financial.
Fill out a separate financial disclosure form for each entity that could be considered a sponsor for this study. This page provides links to commonly used clinical trial forms relevant to clinical trials.