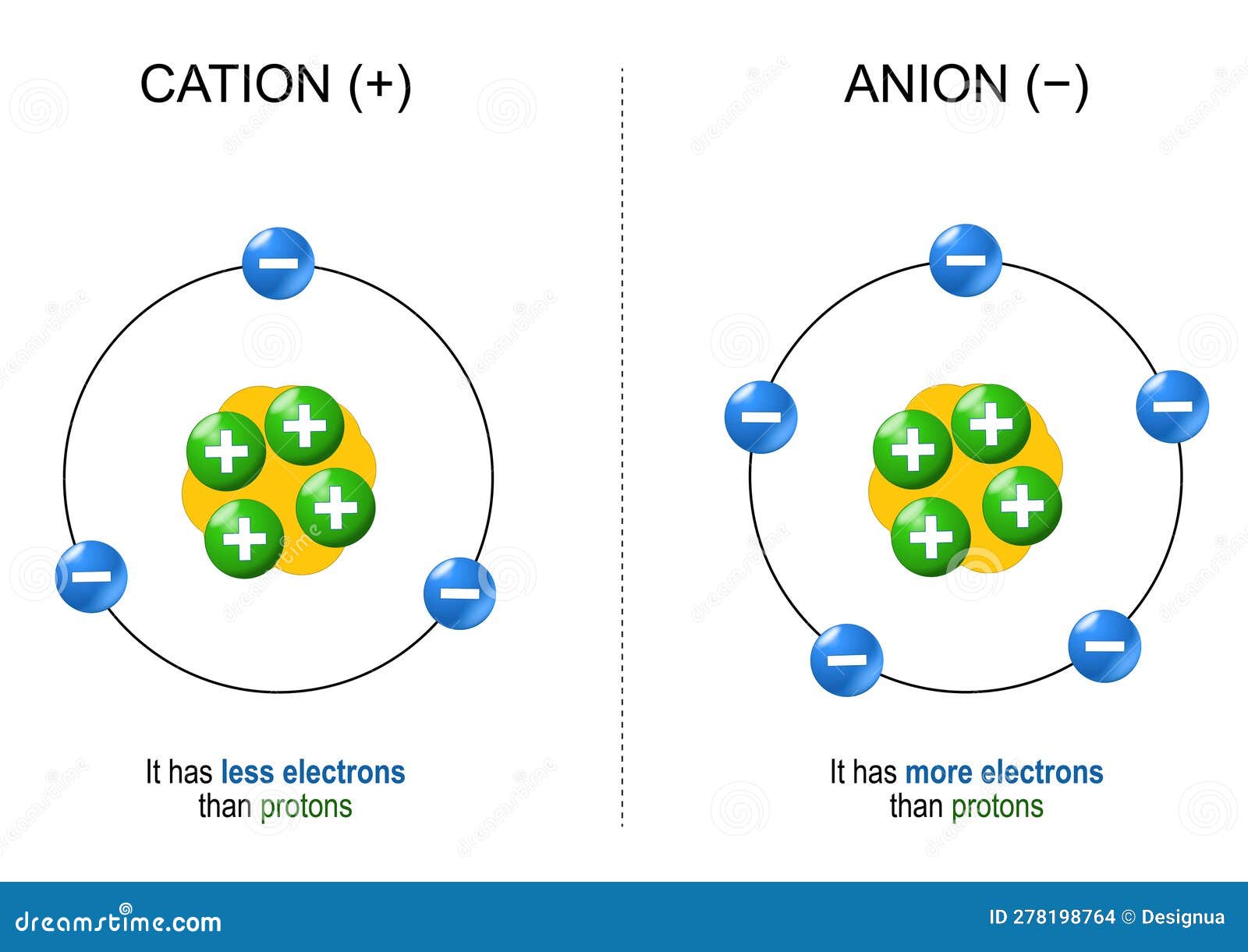
How Is A Cation Formed - The chemical formula of a compound is always written with the cation first, followed by the anion. For a cation to form, one or more. A cation is something that moves down (greek: Summary cations are formed by the loss of one or two electrons from an element. For example, na is the cation and cl is the. For example, in nacl, the sodium atom acts as the. Κάτω, kato, meaning down) and an anion is something that moves up (greek: A cation has more protons than electrons, consequently giving it a net positive charge. When writing the formula of a compound, the cation is listed before the anion. Groups 1 and 2 elements form cations.
A cation is something that moves down (greek: Summary cations are formed by the loss of one or two electrons from an element. The chemical formula of a compound is always written with the cation first, followed by the anion. Groups 1 and 2 elements form cations. For a cation to form, one or more. For example, na is the cation and cl is the. Κάτω, kato, meaning down) and an anion is something that moves up (greek: When writing the formula of a compound, the cation is listed before the anion. For example, in nacl, the sodium atom acts as the. A cation has more protons than electrons, consequently giving it a net positive charge.
Summary cations are formed by the loss of one or two electrons from an element. When writing the formula of a compound, the cation is listed before the anion. A cation is something that moves down (greek: For example, na is the cation and cl is the. The chemical formula of a compound is always written with the cation first, followed by the anion. Κάτω, kato, meaning down) and an anion is something that moves up (greek: For example, in nacl, the sodium atom acts as the. A cation has more protons than electrons, consequently giving it a net positive charge. Groups 1 and 2 elements form cations. For a cation to form, one or more.
Cation Definition, Formation & Examples Lesson
For example, in nacl, the sodium atom acts as the. For a cation to form, one or more. For example, na is the cation and cl is the. When writing the formula of a compound, the cation is listed before the anion. Groups 1 and 2 elements form cations.
Chemistry ppt download
The chemical formula of a compound is always written with the cation first, followed by the anion. When writing the formula of a compound, the cation is listed before the anion. A cation is something that moves down (greek: A cation has more protons than electrons, consequently giving it a net positive charge. Κάτω, kato, meaning down) and an anion.
Ions. ppt download
The chemical formula of a compound is always written with the cation first, followed by the anion. For a cation to form, one or more. A cation has more protons than electrons, consequently giving it a net positive charge. Summary cations are formed by the loss of one or two electrons from an element. When writing the formula of a.
Chemistry I Notes Unit 3 Chapters ppt download
A cation has more protons than electrons, consequently giving it a net positive charge. The chemical formula of a compound is always written with the cation first, followed by the anion. When writing the formula of a compound, the cation is listed before the anion. For a cation to form, one or more. A cation is something that moves down.
What is a Cation Definition of Cation
Summary cations are formed by the loss of one or two electrons from an element. The chemical formula of a compound is always written with the cation first, followed by the anion. A cation has more protons than electrons, consequently giving it a net positive charge. For example, in nacl, the sodium atom acts as the. Groups 1 and 2.
Valence elecrtrons and chemical properties ppt download
For example, na is the cation and cl is the. When writing the formula of a compound, the cation is listed before the anion. Κάτω, kato, meaning down) and an anion is something that moves up (greek: For a cation to form, one or more. The chemical formula of a compound is always written with the cation first, followed by.
List Of Cations
For example, in nacl, the sodium atom acts as the. Groups 1 and 2 elements form cations. For a cation to form, one or more. Summary cations are formed by the loss of one or two electrons from an element. For example, na is the cation and cl is the.
Cations and Anions Definitions, Examples, and Differences
For example, na is the cation and cl is the. When writing the formula of a compound, the cation is listed before the anion. For example, in nacl, the sodium atom acts as the. For a cation to form, one or more. A cation is something that moves down (greek:
Atoms, Molecules, and Ions ppt download
Summary cations are formed by the loss of one or two electrons from an element. When writing the formula of a compound, the cation is listed before the anion. For example, in nacl, the sodium atom acts as the. Κάτω, kato, meaning down) and an anion is something that moves up (greek: A cation has more protons than electrons, consequently.
Structure & Bonding. ppt download
For a cation to form, one or more. Groups 1 and 2 elements form cations. For example, na is the cation and cl is the. A cation has more protons than electrons, consequently giving it a net positive charge. When writing the formula of a compound, the cation is listed before the anion.
The Chemical Formula Of A Compound Is Always Written With The Cation First, Followed By The Anion.
For example, na is the cation and cl is the. Κάτω, kato, meaning down) and an anion is something that moves up (greek: A cation has more protons than electrons, consequently giving it a net positive charge. For example, in nacl, the sodium atom acts as the.
When Writing The Formula Of A Compound, The Cation Is Listed Before The Anion.
Groups 1 and 2 elements form cations. For a cation to form, one or more. Summary cations are formed by the loss of one or two electrons from an element. A cation is something that moves down (greek: