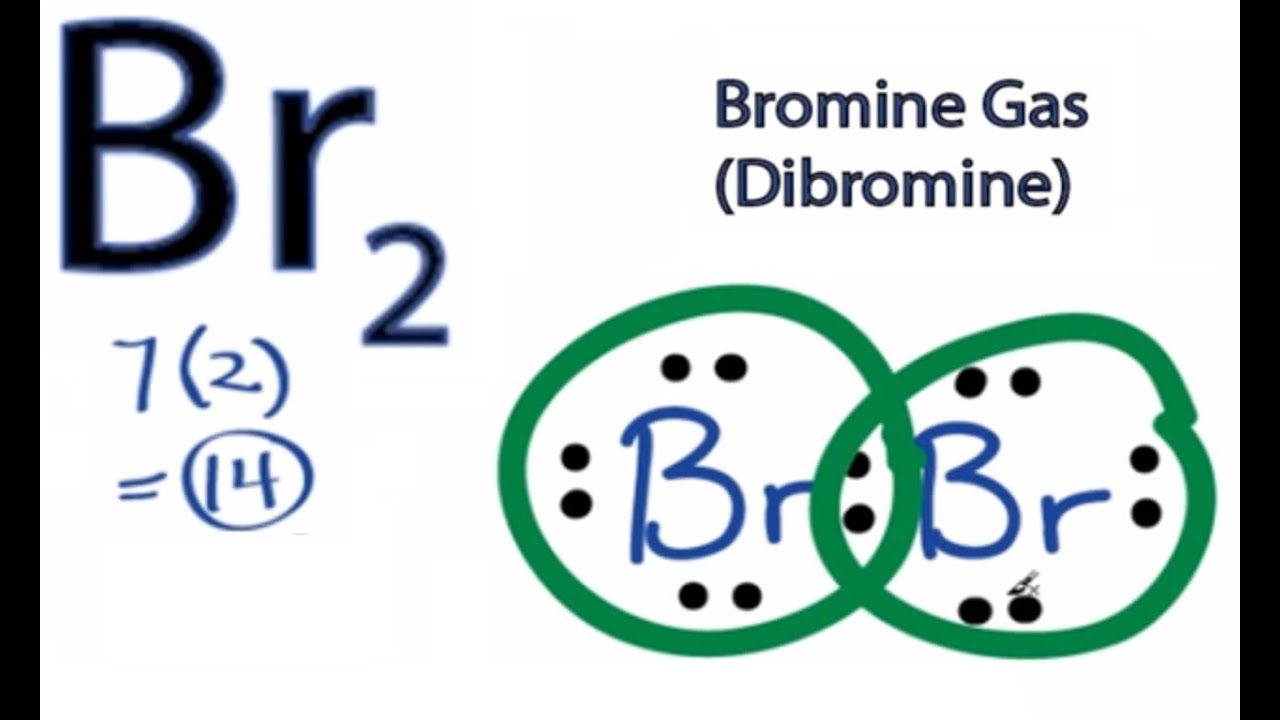
What Is Formed When Two Atoms Of Bromine Bond Together - When two bromine atoms bond, they form a covalent bond by sharing electrons equally, resulting in a stable diatomic bromine molecule. Thus, bromine atoms will typically be found paired. The bond formed between the bromine atoms in a bromine molecule is a covalent bond. When two bromine atoms bond together, they form a diatomic molecule known as bromine (br₂). The bonding occurs through the sharing of electrons in. The two bromine atoms share a pair of electrons, which forms a covalent bond between them. Two atoms of bromine bond together to form a diatomic molecule, specifically br2. In a covalent bond, the atoms share a pair of electrons. This molecule features a nonpolar.
This molecule features a nonpolar. The bond formed between the bromine atoms in a bromine molecule is a covalent bond. Thus, bromine atoms will typically be found paired. In a covalent bond, the atoms share a pair of electrons. When two bromine atoms bond together, they form a diatomic molecule known as bromine (br₂). The bonding occurs through the sharing of electrons in. Two atoms of bromine bond together to form a diatomic molecule, specifically br2. When two bromine atoms bond, they form a covalent bond by sharing electrons equally, resulting in a stable diatomic bromine molecule. The two bromine atoms share a pair of electrons, which forms a covalent bond between them.
Thus, bromine atoms will typically be found paired. In a covalent bond, the atoms share a pair of electrons. The two bromine atoms share a pair of electrons, which forms a covalent bond between them. The bonding occurs through the sharing of electrons in. This molecule features a nonpolar. When two bromine atoms bond together, they form a diatomic molecule known as bromine (br₂). Two atoms of bromine bond together to form a diatomic molecule, specifically br2. The bond formed between the bromine atoms in a bromine molecule is a covalent bond. When two bromine atoms bond, they form a covalent bond by sharing electrons equally, resulting in a stable diatomic bromine molecule.
Ionic and Covalent bonding Chapters 15 and ppt download
When two bromine atoms bond together, they form a diatomic molecule known as bromine (br₂). The bonding occurs through the sharing of electrons in. This molecule features a nonpolar. The bond formed between the bromine atoms in a bromine molecule is a covalent bond. When two bromine atoms bond, they form a covalent bond by sharing electrons equally, resulting in.
Solved Which type of chemical bond is formed between two atoms of
Two atoms of bromine bond together to form a diatomic molecule, specifically br2. This molecule features a nonpolar. Thus, bromine atoms will typically be found paired. When two bromine atoms bond together, they form a diatomic molecule known as bromine (br₂). In a covalent bond, the atoms share a pair of electrons.
Review of Selected Material from Gen Chem I Part I Bonding & Polarity
Two atoms of bromine bond together to form a diatomic molecule, specifically br2. The bonding occurs through the sharing of electrons in. This molecule features a nonpolar. In a covalent bond, the atoms share a pair of electrons. The two bromine atoms share a pair of electrons, which forms a covalent bond between them.
Bromine Atoms Bond Together at Esther Parr blog
This molecule features a nonpolar. The two bromine atoms share a pair of electrons, which forms a covalent bond between them. In a covalent bond, the atoms share a pair of electrons. When two bromine atoms bond together, they form a diatomic molecule known as bromine (br₂). The bonding occurs through the sharing of electrons in.
Chemical Bonds. ppt download
This molecule features a nonpolar. Two atoms of bromine bond together to form a diatomic molecule, specifically br2. In a covalent bond, the atoms share a pair of electrons. The bond formed between the bromine atoms in a bromine molecule is a covalent bond. The bonding occurs through the sharing of electrons in.
Visit For 100’s of free powerpoints ppt download
Two atoms of bromine bond together to form a diatomic molecule, specifically br2. When two bromine atoms bond, they form a covalent bond by sharing electrons equally, resulting in a stable diatomic bromine molecule. The bond formed between the bromine atoms in a bromine molecule is a covalent bond. This molecule features a nonpolar. Thus, bromine atoms will typically be.
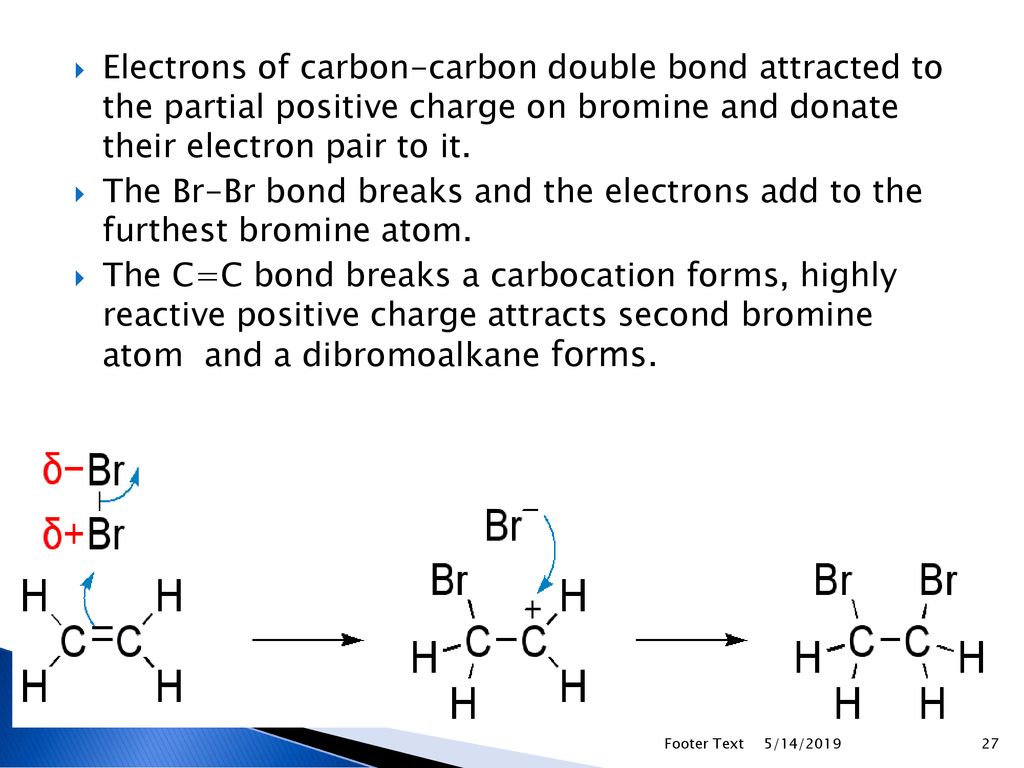
20.1Types of organic reactions ppt download
When two bromine atoms bond, they form a covalent bond by sharing electrons equally, resulting in a stable diatomic bromine molecule. The two bromine atoms share a pair of electrons, which forms a covalent bond between them. This molecule features a nonpolar. The bonding occurs through the sharing of electrons in. Two atoms of bromine bond together to form a.
Draw The Lewis Dot Structure For Bromine at Samantha Brabyn blog
The bond formed between the bromine atoms in a bromine molecule is a covalent bond. In a covalent bond, the atoms share a pair of electrons. The two bromine atoms share a pair of electrons, which forms a covalent bond between them. The bonding occurs through the sharing of electrons in. When two bromine atoms bond, they form a covalent.
Access to Science Chemistry ppt download
This molecule features a nonpolar. The bonding occurs through the sharing of electrons in. When two bromine atoms bond together, they form a diatomic molecule known as bromine (br₂). In a covalent bond, the atoms share a pair of electrons. The two bromine atoms share a pair of electrons, which forms a covalent bond between them.
Bromine Atoms Bond Together at Esther Parr blog
When two bromine atoms bond, they form a covalent bond by sharing electrons equally, resulting in a stable diatomic bromine molecule. The bonding occurs through the sharing of electrons in. Two atoms of bromine bond together to form a diatomic molecule, specifically br2. In a covalent bond, the atoms share a pair of electrons. This molecule features a nonpolar.
This Molecule Features A Nonpolar.
The bonding occurs through the sharing of electrons in. When two bromine atoms bond together, they form a diatomic molecule known as bromine (br₂). Two atoms of bromine bond together to form a diatomic molecule, specifically br2. In a covalent bond, the atoms share a pair of electrons.
The Two Bromine Atoms Share A Pair Of Electrons, Which Forms A Covalent Bond Between Them.
When two bromine atoms bond, they form a covalent bond by sharing electrons equally, resulting in a stable diatomic bromine molecule. The bond formed between the bromine atoms in a bromine molecule is a covalent bond. Thus, bromine atoms will typically be found paired.
+potassium+%26+bromine.jpg)


+Hydrogen..jpg)