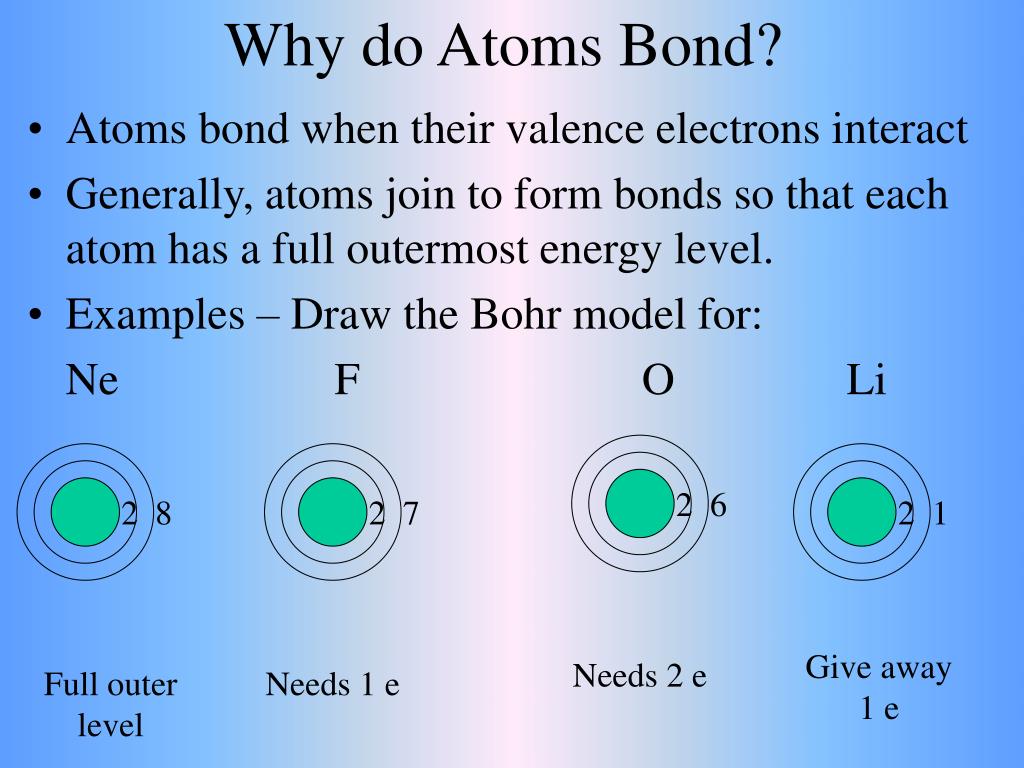
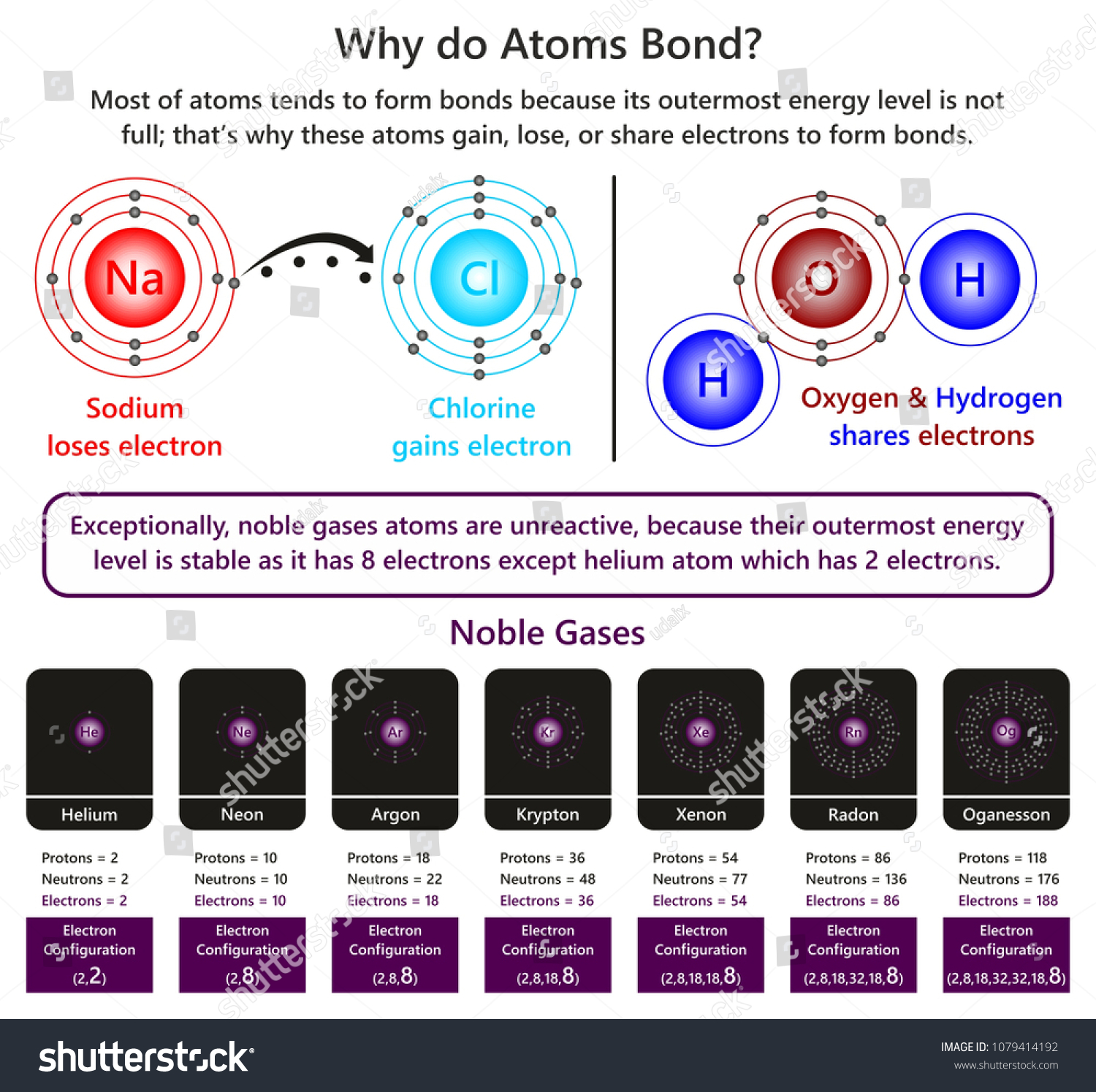
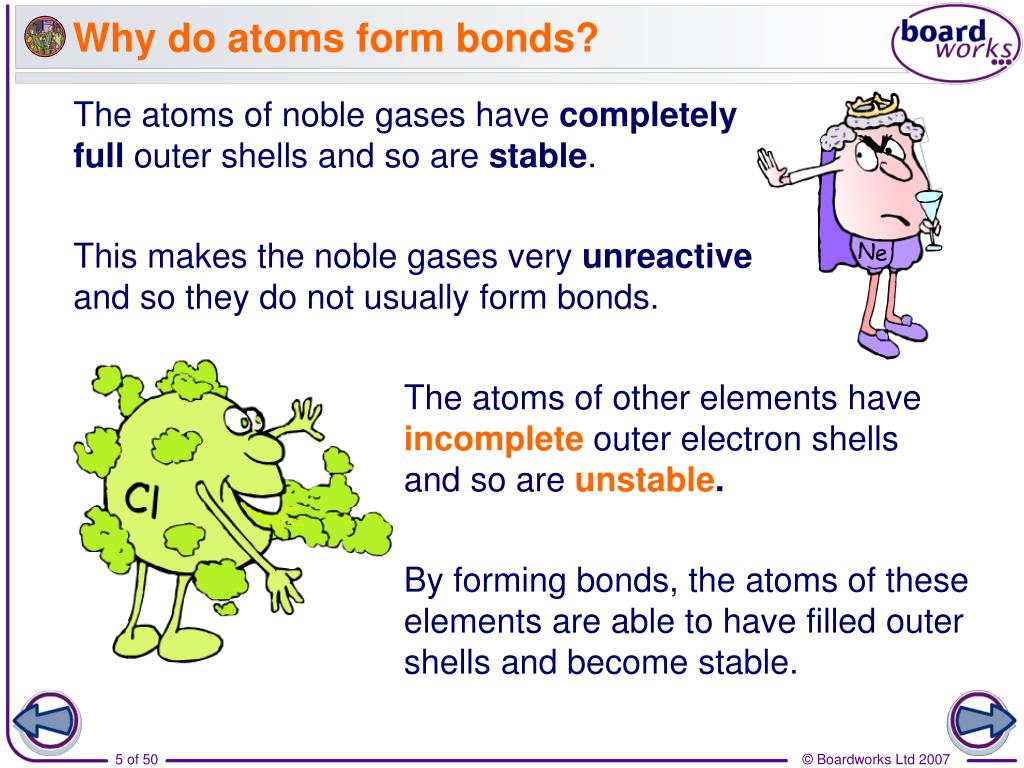
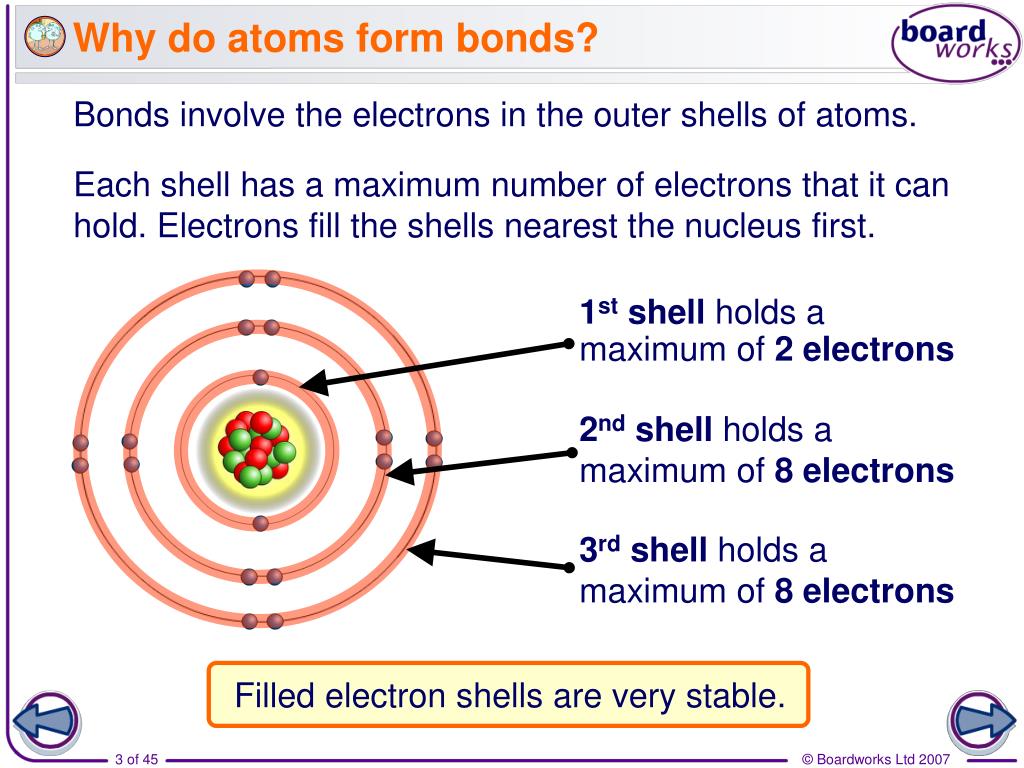
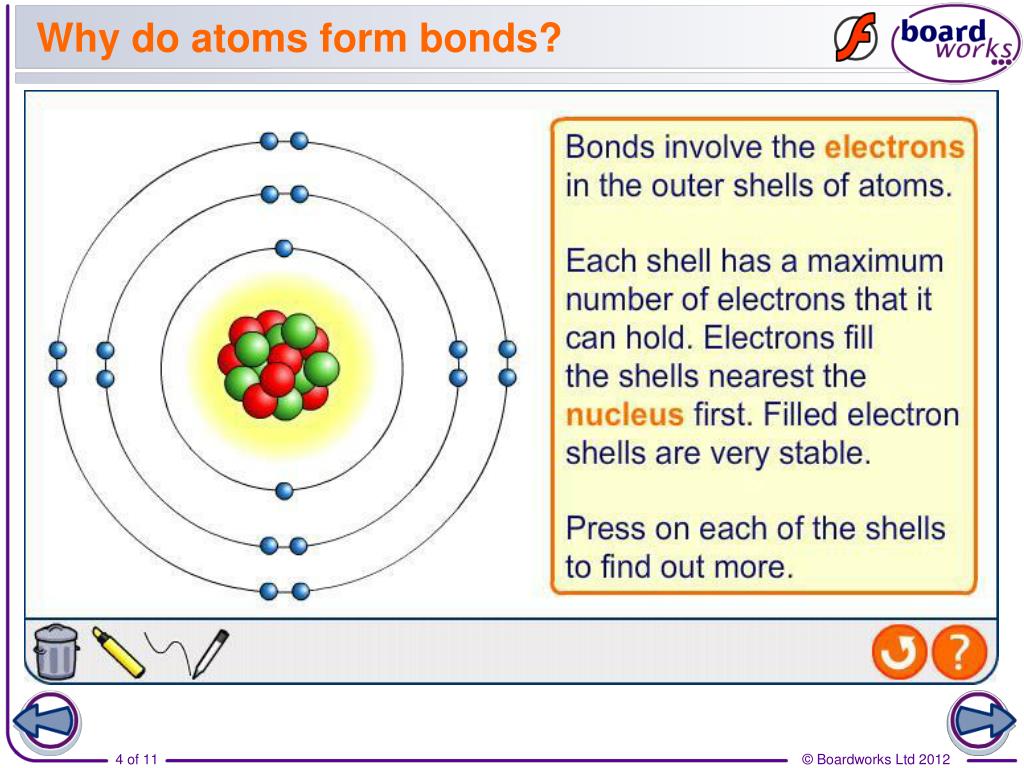
Why Do Atoms Form Bonds - When two atoms form a bond, electrons are donated or shared. The noble gases are called that because they don't form bonds easily. A covalent bond holds the h2o atoms together. In a water molecule, oxygen and hydrogen atoms share electrons to form covalent bonds, resulting in a. This is because they're the most balanced and their outer. In an ionic bond, one atom donates electrons to the other, while in. In these compounds, the atoms are held together by chemical bonds resulting from the sharing or transfer of electrons. Nitrogen atoms will form bonds with other atoms, typically forming covalent bonds with other nonmetals like hydrogen, carbon, and oxygen.
When two atoms form a bond, electrons are donated or shared. In these compounds, the atoms are held together by chemical bonds resulting from the sharing or transfer of electrons. The noble gases are called that because they don't form bonds easily. In an ionic bond, one atom donates electrons to the other, while in. This is because they're the most balanced and their outer. A covalent bond holds the h2o atoms together. Nitrogen atoms will form bonds with other atoms, typically forming covalent bonds with other nonmetals like hydrogen, carbon, and oxygen. In a water molecule, oxygen and hydrogen atoms share electrons to form covalent bonds, resulting in a.
This is because they're the most balanced and their outer. In an ionic bond, one atom donates electrons to the other, while in. In a water molecule, oxygen and hydrogen atoms share electrons to form covalent bonds, resulting in a. The noble gases are called that because they don't form bonds easily. In these compounds, the atoms are held together by chemical bonds resulting from the sharing or transfer of electrons. Nitrogen atoms will form bonds with other atoms, typically forming covalent bonds with other nonmetals like hydrogen, carbon, and oxygen. When two atoms form a bond, electrons are donated or shared. A covalent bond holds the h2o atoms together.
PPT Bonding PowerPoint Presentation, free download ID6007223
When two atoms form a bond, electrons are donated or shared. The noble gases are called that because they don't form bonds easily. In an ionic bond, one atom donates electrons to the other, while in. In these compounds, the atoms are held together by chemical bonds resulting from the sharing or transfer of electrons. Nitrogen atoms will form bonds.
Why do atoms form chemical bonds Online Chemistry Lecture 9th Class
Nitrogen atoms will form bonds with other atoms, typically forming covalent bonds with other nonmetals like hydrogen, carbon, and oxygen. A covalent bond holds the h2o atoms together. In these compounds, the atoms are held together by chemical bonds resulting from the sharing or transfer of electrons. The noble gases are called that because they don't form bonds easily. In.
Why Do Atoms Bond Infographic Diagram stockvector (rechtenvrij) 1079414192
This is because they're the most balanced and their outer. When two atoms form a bond, electrons are donated or shared. The noble gases are called that because they don't form bonds easily. In a water molecule, oxygen and hydrogen atoms share electrons to form covalent bonds, resulting in a. Nitrogen atoms will form bonds with other atoms, typically forming.
PPT Ionic Bonding PowerPoint Presentation, free download ID2664362
A covalent bond holds the h2o atoms together. In a water molecule, oxygen and hydrogen atoms share electrons to form covalent bonds, resulting in a. This is because they're the most balanced and their outer. In an ionic bond, one atom donates electrons to the other, while in. Nitrogen atoms will form bonds with other atoms, typically forming covalent bonds.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Nitrogen atoms will form bonds with other atoms, typically forming covalent bonds with other nonmetals like hydrogen, carbon, and oxygen. In an ionic bond, one atom donates electrons to the other, while in. When two atoms form a bond, electrons are donated or shared. In a water molecule, oxygen and hydrogen atoms share electrons to form covalent bonds, resulting in.
PPT Types of Bonds PowerPoint Presentation, free download ID1996079
This is because they're the most balanced and their outer. In a water molecule, oxygen and hydrogen atoms share electrons to form covalent bonds, resulting in a. In these compounds, the atoms are held together by chemical bonds resulting from the sharing or transfer of electrons. Nitrogen atoms will form bonds with other atoms, typically forming covalent bonds with other.
Chapter 7 Atoms & Bonding ppt video online download
In these compounds, the atoms are held together by chemical bonds resulting from the sharing or transfer of electrons. Nitrogen atoms will form bonds with other atoms, typically forming covalent bonds with other nonmetals like hydrogen, carbon, and oxygen. In a water molecule, oxygen and hydrogen atoms share electrons to form covalent bonds, resulting in a. The noble gases are.
PPT Why do atoms form bonds? PowerPoint Presentation, free download
A covalent bond holds the h2o atoms together. When two atoms form a bond, electrons are donated or shared. In an ionic bond, one atom donates electrons to the other, while in. This is because they're the most balanced and their outer. Nitrogen atoms will form bonds with other atoms, typically forming covalent bonds with other nonmetals like hydrogen, carbon,.
PPT Ions PowerPoint Presentation, free download ID6738771
The noble gases are called that because they don't form bonds easily. This is because they're the most balanced and their outer. In an ionic bond, one atom donates electrons to the other, while in. In these compounds, the atoms are held together by chemical bonds resulting from the sharing or transfer of electrons. A covalent bond holds the h2o.
Ionic and Covalent Bonding Why Do Atoms Form
In these compounds, the atoms are held together by chemical bonds resulting from the sharing or transfer of electrons. In a water molecule, oxygen and hydrogen atoms share electrons to form covalent bonds, resulting in a. A covalent bond holds the h2o atoms together. This is because they're the most balanced and their outer. When two atoms form a bond,.
In A Water Molecule, Oxygen And Hydrogen Atoms Share Electrons To Form Covalent Bonds, Resulting In A.
In an ionic bond, one atom donates electrons to the other, while in. Nitrogen atoms will form bonds with other atoms, typically forming covalent bonds with other nonmetals like hydrogen, carbon, and oxygen. A covalent bond holds the h2o atoms together. When two atoms form a bond, electrons are donated or shared.
In These Compounds, The Atoms Are Held Together By Chemical Bonds Resulting From The Sharing Or Transfer Of Electrons.
The noble gases are called that because they don't form bonds easily. This is because they're the most balanced and their outer.